Type-2 diabetes mellitus (T2DM), the predominant form of diabetes in adults, is a co-morbid condition that exacerbates the severity of many other diseases, including cardiovascular disease, obesity, dyslipidemia, hypertension, and cancer. Among these, cancer is particularly concerning due to elevated mortality rates and a distinct lack of cost-effective therapeutic interventions. Identifying novel biomarkers for improved early cancer detection is imperative. Therefore, an integrated bioinformatics analysis was conducted to elucidate the co-morbid relationship between T2DM and five different types of cancer, namely bladder (BLCA), breast (BRCA), colon (CRC), liver (HCC), and prostate cancer (PRAD) and identification of novel biomarkers for early cancer detection in individuals with T2DM. A significant comorbid relationship was observed among T2DM, BLCA, and BRCA through gene expression and pathway enrichment analysis, while a moderate association was observed for between T2DM, and PRAD. Notably, we identified 18 significant hub proteins in the context of cancer and T2DM, along with 16 transcription factors and 5 miRNAs. Among these, the hub proteins ESR1, PIK3CA, GNAI1, ERBB2, NR3C1, SNCA, TGFBR2, as well as the micro RNAs hsa-mir-335–5p, hsa-mir-16–5p, and hsa-mir-93–5p hold promise for understanding the comorbidities of T2DM and cancers; and could serve as valuable disease biomarkers for clinical diagnosis and prognosis. This study, centred on bioinformatics analysis for biomarker identification in comorbidities, paves the way for future research encompassing wet lab experimentation and translational studies. These endeavours are poised to validate and facilitate the integration of these findings into the realm of personalized medicine.
Probing the Network Between Cancer and Type-2 Diabetes
An Advanced Computational Paradigm for Biomarker Screening
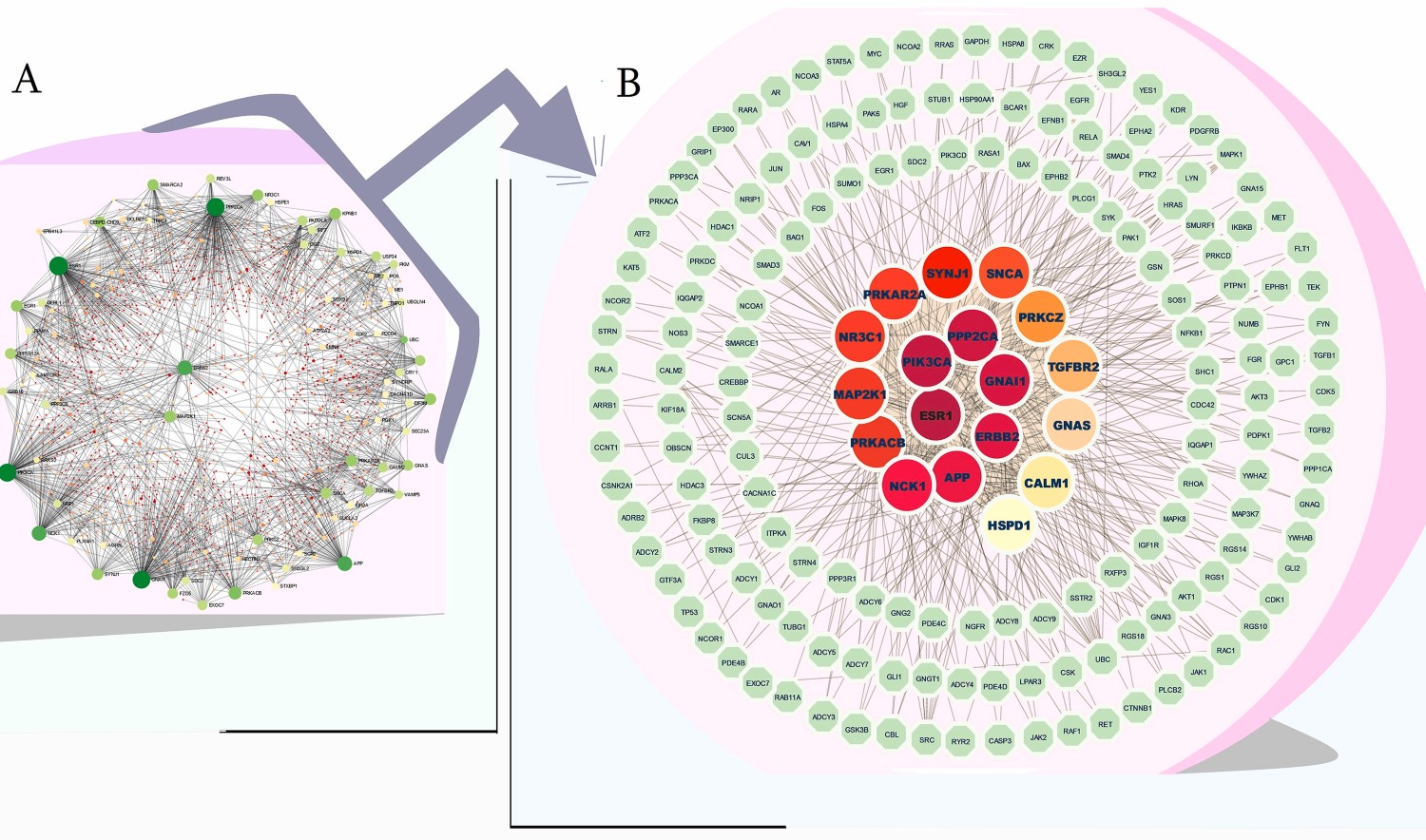
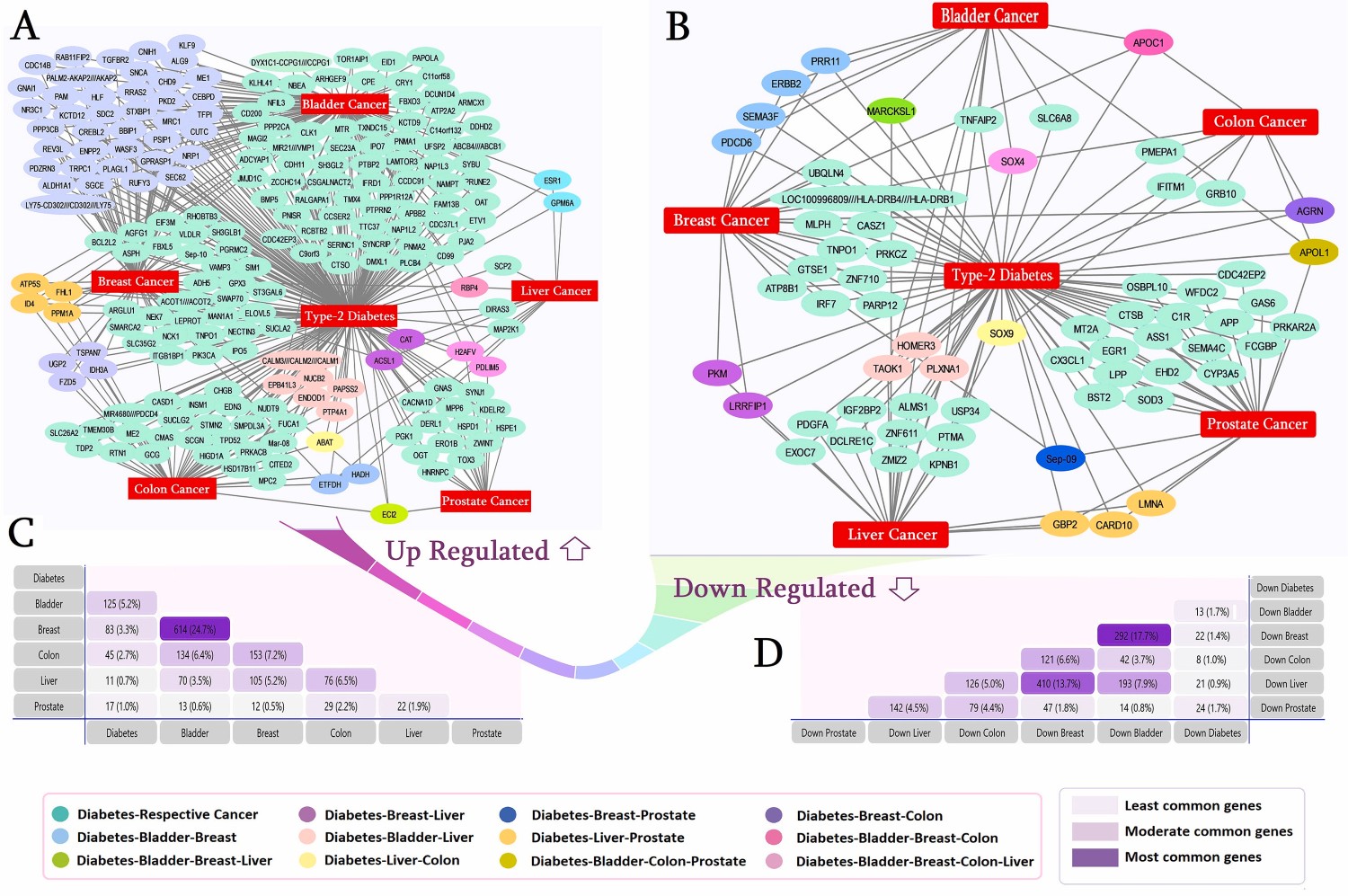
Discussion
Cancer remains a leading cause of mortality worldwide, with its severity often compounded by comorbidities like diabetes mellitus. Individuals with Type 2 Diabetes (T2DM) face a significantly higher risk—up to 1.5 times—of cancer-related death from colorectal, pancreatic, liver, and endometrial cancers compared to the non-diabetic population. While epidemiological studies have long confirmed these associations, the precise molecular connections have remained a puzzle.
This study leveraged integrated bioinformatics to bridge this knowledge gap, analyzing the complex interplay between T2DM and five prevalent cancers: Bladder (BLCA), Breast (BRCA), Colon (CRC), Liver (HCC), and Prostate (PRAD). By executing rigorous statistical analysis on publicly available gene expression datasets, we aimed to uncover the underlying molecular interactions that drive these comorbidities. The analysis revealed a significant number of differentially expressed genes shared between T2DM and the selected malignancies. Notably, the SOX4 protein, which regulates insulin secretion and is involved in tumor progression, was found to be downregulated across T2DM, BLCA, BRCA, HCC, and CRC, highlighting a potential common link.
Further investigation into shared molecular pathways identified several critical connections. For instance, the renowned estrogen signaling pathway was found to link T2DM with cancers, particularly breast cancer. In T2DM, hyperinsulinemia can lower sex hormone-binding globulin, increasing the bioavailability of estrogen and consequently raising the risk of BRCA. Similarly, fat metabolism pathways were strongly associated with BRCA, HCC, and CRC comorbidities. This study identified 18 central "hub proteins" that form the core of the T2DM-cancer network, with ESR1 being the most significant link, particularly in breast, bladder, and prostate cancers. Other key proteins like PIK3CA and ERBB2 also showed significant dysregulation. The investigation also pinpointed five pivotal microRNAs (including hsa-mir-335–5p) and 16 transcription factors that regulate the expression of these shared genes. These findings not only provide valuable insights into the intricate relationship between diabetes and cancer but also offer a validated set of potential biomarkers—such as ESR1, PIK3CA, and specific miRNAs—that can pave the way for future research and the development of targeted, personalized medicine.
